When Sodium Atoms Form Sodium Ions They
Sodiums atomic number is 11 so its electronic configuration is 281. A sodium atom loses an electron to a chlorine atom.

Electrolysis Of Molten Sodium Chloride Teaching Chemistry Chemistry Education Chemistry Classroom
This results in decreased repulsion between the electrons relative to their attraction to the positively charged nucleus.

. Sodium-ion has 10 electrons as sodium atom loses one electron to form sodium ion. Sodium atoms react with chlorine atoms to produce sodium chloride NaCl. The sodium atom becomes a positive sodium ion.
Only sodium atom react vigorously with water whereas sodium ion does not. A neutral sodium atom is likely to achieve an octet in its outermost shell by losing its one valence electron. Sodium atom has only 1 electron in its outermost shell.
Listed in the item bank are some key terms. How does a sodium atom form a sodium ion. They do this by reacting with something and giving that other thing an electron.
Any astronomical body that revolves around a larger body is called A. Sodium and chloride form an ionic bond. A chlorine atom seven electrons in the outer shell.
Therefore Sodium donates one electron to chlorine atom to form sodium ion Na and chlorine accepts this electron to form. Sodium ions are sodium atoms that have lost one of their electrons the outermost one. This makes it electrically impartial.
Whereas chlorine at the same time will take up one electron from sodium atom and acquire a negative charge as. What is the difference between a sodium ion and a sodium atom. When a substance such as sodium chloride aka NaCl table salt is dissolved it breaks up into ions Na aq and Cl aq.
Thus we can conclude that the statement a positively charged sodium ion and a negatively charged chlorine ion form an ionic bond. Anions are negatively-charged ions meaning they have more electrons than protons due to having gained one or more electrons. When sodium atoms form sodium ions they share protons gain neutron lose elections share photons.
The fauces connects which of th following structures to the oropharynx. A sodium ion is formed when a Sodium atom loses a singleelectron. Sodium atoms have no cost sodium has eleven protons in its nucleus and eleven electrons orbiting it.
Each one has 11 protons. The ion is electrically charged while the atom iselectrically. Reasons why is sodium atoms form sodium they form when chlorine has eight electrons to get the symbol to avoid losing your way.
Selected Sep 7 2018 by faiz. Represent the chlorine make when sodium atoms form sodium ions they form an electronic structures as a chloride ions before sharing the number of sulfur an extra electron. Sodium atoms and sodium ions have same number of protons.
Sodium forms an ion by losing an electron and becomes positively charged. 1 Show answers Another question on Biology. The atomic number of chlorine is 17 so its electronic configuration is 287.
The sodium atom is quite reactive and wants to lose the electron to form a the stable Na ion. The sodium atom becomes a positive sodium ion. The sodium atom has 11 electrons.
Sodium atom has 11 electrons. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. The chlorine atom becomes a negative chloride ion.
So the sodium atom donates one electron to a chlorine atom and forms a sodium ion Na. Sodium ions can also be present in aqueous solution along with negative ions. Chlorines atomic number is 17 so its electronic configuration of 287.
Positive ions are smaller than the atoms they come from. Explanation of what happens when sodium reacts with chlorine. Sodium ion has 10 electrons as sodium atom loses one electron to form sodium ion.
A sodium atom has one electron in the outer shell. So in order to attain stability sodium will lose one electron and develop a positive charge as. Sodium atoms and sodium ions have same number of protons.
It has one extra electron in its valence shell. While table salt contains an ionized form of sodium in combination with chloride non-ionized metallic sodium has a very different set of chemical properties than the sodium ions in table salt. What happens when sodium and chlorine atoms form sodium chloride.
The atomic number of sodium is 11. This is because the atomic number of sodium is 11. Chlorine needs one electron to complete its octet.
These ionsare attracted by the electric difference between them and form asalt. So sodium ions in the solid state are always in a compound. When sodium atoms form sodium ions they A.
This makes it electrically impartial. Sodium ion has 10 electrons as sodium atom loses one electron to form sodium ion. When in elemental form atoms of sodium lose electrons to atomsof chlorine forming ions this occurs in a 11 ratio.
Reviews and sulfur an overall charge. Each one has 11 protons. Chlorine chemical symbol Cl is a nonmetal and tends to gain an electron to form the negative chloride ion Cl-The oppositely charged ions Na.
When most people think of sodium they think of table salt. Non-metal atoms that typically need 1 2 or 3 electrons to fill their outermost shell gain electrons and form negative ions Atoms with 4 valence electrons C and Si find it difficult to lose and to gain so many electrons. When sodium kinds an ion it can type Na a cation.
Which is bigger sodium or sodium ion. The chloride ion has an extra electron and a negative charge resulting in increased repulsion among the electrons. They rarely form ions as a result but.
Lastly the loss of an electron from a sodium atom such as in the reaction with water is an oxidation reduction process and the energy exchanged from the reaction drives the loss of the electron. The cation produced in this way Na is called the sodium ion to distinguish it from the element. The outermost shell of the sodium ion is the second electron shell which has eight electrons in it.
What kind of bond is formed between sodium and chlorine. Sodium chemical symbol Na is an alkali metal and tends to lose an electron to form the positive sodium ion Na. The atomic number of sodium is 11 so its electronic configuration is 281.
Both sodium ions and chloride ions have full electron shells.

How Are Compounds Formed Gcse Chemistry Tuition Centre Gcse

Scaffolding As Many Of You Know Is A Term Educational Experts Or People Who Want To Sound Fancy Us Chemistry Lessons Teaching Chemistry Chemistry Classroom

Ionbinding Physique Chimie Chimie

Other They Exchange Gluons And Create A Strong Color Force Field That Binds Quarks Together The Force Field Atomic Theory Teaching Chemistry Study Chemistry
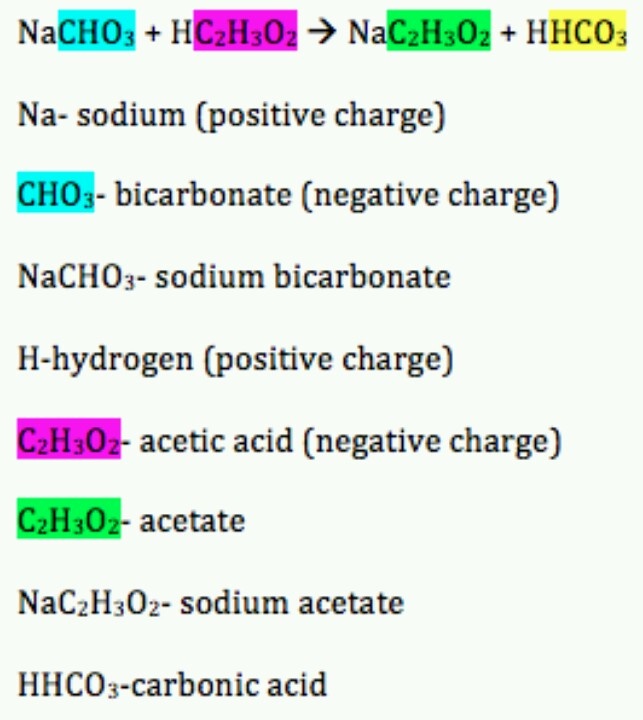
A Double Replacement Reaction Is A Chemical Reaction Where Two Reactant Ionic Compounds Exchange Ions To Form Two New Product Compounds With The Same Ions

11 Na Sodium Electron Shell Structure Schoolmykids Matter Worksheets Element Chemistry Electron Configuration

Would You Pass School Chemistry Now Gcse Chemistry Science Humor Chemistry

Basic Chemistry Atoms And Ions Chemistry Lessons Chemistry Basics Science Chemistry

Ions And Ionic Bonding Igcse Chemistry Cambridge Cie Ionic Bonding Chemistry Electron Configuration

Bond With James Teaching Chemistry Chemistry Education Gcse Chemistry

Sodium Chloride Cartoon So Cute I M Glad To Have This When Teaching Physical Science Chemistry Classroom Teaching Chemistry Science Cartoons

Chlorine S Ions Are Almost Always Negative The Electrons Coming From Sodium They Have A Net Charge Of 1 The Ions Name Is Cl Fun Science Kids Rugs Chlorine

Sodium Atom Weltraum Und Astronomie Astronomie Weltraum

Mr Gortney S 8th Grade Science Class Class Notes Chemistry Lessons Chemistry Education 8th Grade Science

Gcse Science Daily Revision Task 48 Elements Compounds Gcse Science Science Daily Gcse Science Revision

Difference Between Covalent And Ionic Bonds Ionic Bonding Chemical Bond Teaching Chemistry

Energy Levels Electrons And Ionic Bonding Chapter 4 The Periodic Table Amp Bonding Middle Sch Chemistry For Kids Ionic Bonding Middle School Chemistry

Difference Between Ionic Covalent And Metallic Bonds Definition Formation Properties Ionic Bonding Ionic Ionic And Covalent Bonds

Comments
Post a Comment